 In June 2006, the anti-inflammatory analgesic drug diclofenac was banned for veterinary use in Nepal, because vultures were dying after ingesting it from livestock carcasses. But a ban must be monitored. Conservation experts say diclofenac is still on the shelves, and that illegal production and import continues.
In June 2006, the anti-inflammatory analgesic drug diclofenac was banned for veterinary use in Nepal, because vultures were dying after ingesting it from livestock carcasses. But a ban must be monitored. Conservation experts say diclofenac is still on the shelves, and that illegal production and import continues.
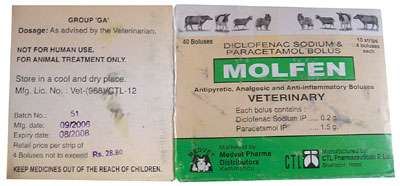
CTL Pharmaceuticals of Bhaktapur continues to produce the drug under the name Molfen, says BCN. A recent survey by Bird Conservation Nepal (BCN) found that CTL's brand of diclofenac, Molfen, was still being sold in veterinary drug stores in Banke, Kanchanpur, Rupendehi, Dang, and Kailali.
"We have proof to show that Molfen is still being produced," says Hem Sagar Baral, chief executive of BCN. Molfen packaging in the possession of BCN has dates of production as recently as September 2006.
"Why is the government not going after people involved in the production and import of an illegal drug?" asks Baral. Baral says it will take a few decades for the vulture population to come back to the size it was 25 years ago-if deaths such as those from diclofenac are stopped.
Seven years ago, when the vultures in South Asia suddenly started dying, at different times the use of pesticides and lack of food were blamed. In late 2003, American researchers in Pakistan found the doclofenac connection. At a meeting in Kathmandu in February 2004, South Asian countries pledged to ban the drug. A new non-toxic drug, meloxicam, found to be safe for livestock and vultures, was to be administered instead. A month after the ban came into effect in Nepal, diclofenac producer Medivet started manufacturing meloxicam instead.
Mallika Aryal
Related stories:'Soaring again' #286 and 'No more circling' #185.


